Why is Medical Technology Innovation so Hard?
Medical technology innovation has dramatically expanded, especially during the COVID-19 lockdown. Mobile apps were used to track and trace to spread of the pandemic, and video chatting apps replace physical doctor appointments. This extent highlighted several medical technology benefits for the healthcare system, such as noninvasive treatments, accessible services and patient-centric solutions. Unlike pharmaceutical or surgical interventions, medical technology can provide less painful and risk-free alternatives, especially for patients with chronic diseases.
Along with the vast opportunity that innovative medical technology can provide for patients, several challenges face companies working in the med-tech field. These barriers stand against the benefits that it can provide for its patients. In this article, I will try to shed light on some of the obstacles that face companies, especially SMEs, which present the majority of enterprises working in this field. Also, I would provide insights to address these challenges inspired by Sir George Cox’s review: Review of Creativity in Business: Building on the UK’s Strengths.
Related articles:
- Tips to Achieve Patient-Centred Health Technology
- Five Reasons to Utilise Patient-Centric Medical Innovation
To address healthcare challenges, the current business models need renovation and a shift toward patients’ needs. Achieving this target can only happen with technological and business innovation. As highlighted by TimBrown, innovation requires three pillars: human needs, technology feasibility, and business viability. During the COVID-19 pandemic, the shifted toward health technology (Figure 1) presented one of the tools that the healthcare system used to overcome the lockdown barriers and reduce face-to-face contact, mainly with vulnerable patients (Four Reasons to Expand Mobile Technology Usage in Medical Innovation).
Although the several benefits of med-tech, it is not widely adopted in the medical sector and facing challenges. Barriers may vary from one healthcare system to another, yet small and medium enterprises (SMEs) face general challenges. The below video shows
What is Medical Technology?
Before jumping to explore medical technology barriers, there should be a clear definition of the medical technology covered in this article. Generally, medical technology applications extend to medical and surgical instruments and facilities. In some definitions, drugs are listed as medical technology. The Med-Tech involves various applications related to patient’s experience, such as disease prevention, diagnosis, monitoring and treatment (Figure 2). Medical technology administration can be full by clinicians, patients and clinicians, or entirely administered by patients.

n other definitions, health technology may hold a broader meaning to cover human healthcare overall such as weight loss, while medical technology focuses on the diagnosis, monitoring, and treatment. In this article, both terms are used interchangeably to refer to the technology used to expand patient empowerment. This article will focus on the self-administered medical technology innovation, and the reason is that patient adherence adds another layer of challenges compared with clinically-administered intervention. When patients use the medical technology at home, the results are blurred by poor adherence, which affects the ability to measure the actual impact of the intervention. Low adherence can fall under 40% in chronic diseases such as cardiovascular diseases and diabetes. The video below presents innovative medical technology ideas for the future:
Barriers to Medical Technology Innovation
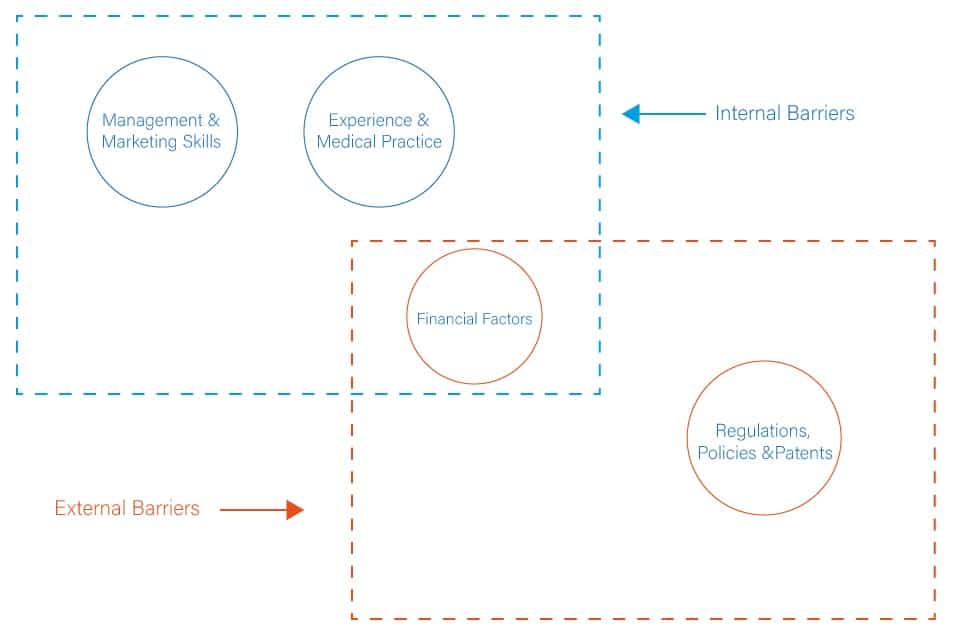
The barriers that face medical technology SMEs are a mix of general business barriers encountered by most SMEs and barriers related to the healthcare system’s nature. Overall, the barriers can be categorised as internal and external (Figure 3). The internal factors include the following:
Management and Marketing Skills
Running medical projects requires more than managerial skills. Both innovation management and technical skills are needed to run the project. The lack of necessary management skills is behind the failure of many SMEs. Another challenge is when the company runs multiple projects at the same time. Companies’ common thinking focuses on straightforward and granted successful projects on the cost of high-risk, innovative ideas. Without having a place for innovation in a firm’s strategic planning, innovative projects would be affected by rapid granted-win projects.
Another side of managerial skills is attracting adequate employees and managers with the relevant training and appreciation for the medical technology innovation. The resistance to change and innovation can be a result of inadequate employees training and skills. On the managerial level, skilled managers with an excellent ability to adopt an innovation strategy can drive a successful innovation profile. In contrast, managers lacking innovative skills hinder SMEs from achieving innovation even if they have the necessary innovative and creative skills.
Studies have shown while SMEs focus on the value proposition of their product or service, technical and innovation skills come before marketing skills required for the project success. The poor marketing strategy and financial planning for these marketing activities stand as barriers between companies’ innovative ideas and access to their consumers, especially when patients can buy the product directly from shelves and full self-administrate the regimen.
Experience and Medical Practice
The medical sector is influenced by scientific research and how it drives the medical practice to adopt innovative solutions. This relation between the medical industry and innovation is problematic from the perspective of driving innovation. Companies need to conduct several clinical trials and meta-analysis studies to drive the medical bodies to acknowledge innovative medical technology. After achieving this recognition, the new solutions take a long time to be adopted on an industrial scale to cover the production costs, clinical trials and related studies. This process is an effort, cost, and time-consuming, especially for companies who lack the experience of the medical practice’s unique nature.

Another side of the experience barriers is the lack of technical knowledge required for the new product development, such as the inadequate knowledge of the targeted healthcare condition. This lack of experience negatively impacts the medical technology intervention itself, the development process, and access to the market. From the evaluation perspective, the lack of experience affects the setup protocols and guidelines for clinical trials and related studies and the preciseness of the setup protocols associated with these studies.
In addition to the above internal factors, external factors affect SMEs’ ability to drive medical technology innovation in the market. These factors include the following:
Financial Factors
By asking companies about their problems, financial barriers come at the top of the list, especially as a significant hindrance to SMEs’ innovation. The financial barriers can be either external or internal inside the SMEs. The top financial obstacle that faces companies is the cost of the clinical trials, meta-analysis studies and other studies required to drive the medical sector to adopt the new solution. Additionally, financial problems include capitalisation, short-term liquidity problems, insufficient working capital, insufficient start-up capital, and poor financial management. These reasons put the financial barriers in both internal and external factors. Other financial problems include high monitoring costs and the fund providers’ inability to adequately assess the project, especially in innovative medical solutions with a high-risk profile.
In medical technology companies, the financial problems are magnified by the sector’s nature as companies take a long road through different regulations until their products can be evaluated and approved in the market. While funding opportunities allow companies to go through the innovation process, the funding may not help companies sustain through the process without effective financial management.
Regulations, Policies and Patents
Overall, the regulation to access national and international markets involves complex, lengthy, cost and sometimes blurry process such as the CE mark. The level of regulation complexity is associated with the classification of the medical product. The higher the device’s class, the more complex process to penetrate markets through the FDA in the US, Notified Bodies in the EU and NHS in the UK. The complex regulations and policies are considered major hinders that stands between companies and accessing different markets.
Another barrier is the patent of the medical technology itself. Medical innovation SMEs’ biggest asset is their product or service idea. Registering a patent is complex and requires the company to have experience or hire someone with the knowledge, which is an expensive process. The cost of patents increases based on the number of countries and regions where the medical device needs to be registered. The more markets that the product is protected, the more the patent cost on the company.
Poor Patient Adherence
One of the advantages of medical technology innovation is providing self-administered solutions that patients can use at home without being admitted to the hospital or attending a doctor’s appointment. However, poor adherence is a significant problem that falls under 40% in chronic diseases where the patient is required to take the treatment for several months or even years. Poor adherence can also mask the efficiency of the treatment technology during clinical trials because patients are simply not taking the treatment as prescribed. The complex nature of adherence presents a challenging element in developing medical technology innovation. Additionally, there was no practical framework that allowed the companies to evaluate their consideration for adherence. My research during the PhD journey involved this point leading to the development of the adherence canvas, a tool for companies to consider adherence during health technology development. The below video shows how Adherence Canvas works, and it can be downloaded from the main website, Adherence Canvas.
Driving the Medical Technology Innovation Forward
There are several solutions that can drive innovation in med-tech companies such as the Double Diamond design thinking process. However, the focus on the business, technology and human factors highlighted by Tim Brown can provide the companies with a clear idea about the business and how companies can build a solid value proposition in the market. This internal design for the business helps companies to establish based on solid ground. However, it doesn’t provide the sufficient financial and technological support to progress. Therefore, Sir George Cox highlighted several points to support SMEs in his report: Cox Review of Creativity in Business: Building on the UK’s Strengths. He highlighted number of procedures to be adopted by the British government in order to establish competing creative economy through supporting SMEs in general. These procedures include:
Raising Awareness and Changing Behaviour
In this part, the plan involves a national-wide programme that increases the awareness of creativity and innovation in business, which presents the knowledge part. Expanding this knowledge can help companies overcome the technical knowledge barriers and provide them with the support that can drive them to move forward with their production process.
Providing Support and Incentives
Along with the awareness plan, the strategy will focus on introducing supportive laws and procedures for companies and start-ups. These regulations can include providing tax benefits for enterprises that apply creativity and innovation. Another type of support is to help the companies to establish their profile and providing support when establishing the companies.
As I worked with several start-ups and SMEs throughout my research, I found significant support from health-related organisations in the UK. These organisations can provide financial and technical help for companies such as the Academy of Health Science Network (AHSN) and the National Institute of Health Research (NIHR), which has divisions across the country. Also, large enterprises can provide funding and support for start-ups, such as the Centre of Process Innovation (CPI). Innovate UK includes all the funds called in several sectors, so it can be your first stop if you like to search for funding to start your business. Another type of funders is related to specific diseases, such as Diabetes UK and the British Heart Foundation.
Conclusion
Medical technology innovation is one of the rapidly growing sectors. The importance of this sector has expanded after the COVID-19 pandemic. However, SMEs are facing significant barriers and challenges that stand between them and developing new technologies. These barriers can be either internal or external. While medical technology innovation benefits are enormous, healthcare systems should help these SMEs by funding and experience support, easing regulations, and providing policy support.
Medical technology innovation’s impact is magnified in chronic diseases and patients who self-administer their treatment regimen due to the intervention’s long-term usage. Medical technology can reduce costs, drive patient-centric technology, and reduce the dependency on pharmaceutical drugs and invasive treatments. While several barriers face companies, several funders and organisations can support companies, especially in the early stages.
Bibliography
- Bergsland, J., Elle, O. J., & Fosse, E. (2014). Barriers to medical device innovation. Medical Devices. 7. p. 205–209.
- David, Y., Judd, T. M., & Zambuto, R. P. (2020). Introduction to medical technology management practices. In Clinical Engineering Handbook (pp. 166-177). Washington D.C.: Academic Press.
- Garrett, P., Brown, C. A., Hart-Hester, S., Hamadain, E., Dixon, C., Pierce, W., & Rudman, W. J. (2006). Identifying barriers to the adoption of new technology in rural hospitals: a case report. Perspectives in Health Information Management. 3. p.9.
- National Research Council. (2002). Medical innovation in the changing healthcare marketplace: Conference summary. Washington D.C.: Academy Press





I like when you say that medical technology innovations benefit the healthcare system and impact medical technology. This way, there will be a medical program that can create awareness of creating innovative devices and medical equipment. If we continue to build the technology, I believe we can make a significant change in improving the medical field.
Yes, this is the aim. Thanks!